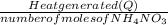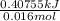Chemistry, 25.03.2020 02:03 AkramMasoud
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and 4.18 J/(g⋅∘C) as the specific heat capacity.) Express the enthalpy change in kilojoules per mole to two significant figures. ΔHrxn = nothing kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and...
Questions



Mathematics, 26.01.2020 11:31

Social Studies, 26.01.2020 11:31

Mathematics, 26.01.2020 11:31



Mathematics, 26.01.2020 11:31

Mathematics, 26.01.2020 11:31

Mathematics, 26.01.2020 11:31

History, 26.01.2020 11:31




History, 26.01.2020 11:31


Biology, 26.01.2020 11:31

World Languages, 26.01.2020 11:31


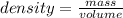

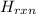 =
=