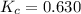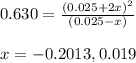Chemistry, 25.03.2020 02:08 gunnatvinson
The value of kc for the following reaction is 0.630 at 409 K N2O4(g) --> 2NO2(g) if a reaction vessel at that temperature intitially contains 0.0250 M NO2 and 0.0250 M N2O4, what is the concentration of NO2 at equilibrium

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2


Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
The value of kc for the following reaction is 0.630 at 409 K N2O4(g) --> 2NO2(g) if a reaction ve...
Questions

Mathematics, 05.10.2020 04:01

Mathematics, 05.10.2020 04:01

History, 05.10.2020 04:01

Mathematics, 05.10.2020 04:01



Mathematics, 05.10.2020 04:01

Mathematics, 05.10.2020 04:01

Mathematics, 05.10.2020 04:01

Mathematics, 05.10.2020 04:01


Mathematics, 05.10.2020 04:01

Biology, 05.10.2020 04:01





English, 05.10.2020 04:01


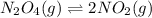
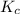 for above equation follows:
for above equation follows:![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0562/2368/271f5.png)