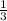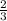Chemistry, 25.03.2020 01:21 FavvBella84
The total pressure for a mixture of N2O4 and NO2 is 1.5atm. If Kp = 7.1 at 25°C, calculate the partial pressure of each gas in the mixture. (HINT 1.5 = 2pNO2 + pN2O4) 2NO2 (g) = N2O4 (g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2


Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
The total pressure for a mixture of N2O4 and NO2 is 1.5atm. If Kp = 7.1 at 25°C, calculate the parti...
Questions

Chemistry, 14.11.2020 01:00




Biology, 14.11.2020 01:00

Chemistry, 14.11.2020 01:00


Mathematics, 14.11.2020 01:00

Chemistry, 14.11.2020 01:00

English, 14.11.2020 01:00


Chemistry, 14.11.2020 01:00


English, 14.11.2020 01:00

Physics, 14.11.2020 01:00




History, 14.11.2020 01:00

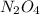 : 0.5 atm and
: 0.5 atm and 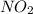 : 1 atm
: 1 atm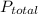 = 1.5 atm
= 1.5 atm