
Chemistry, 25.03.2020 01:35 naiomireyes74p2aybs
An element has the following successive ionization energies: I1 = 578 kJ/mol I2 = 1817 kJ/mol I3 = 2745 kJ/mol I4 = 11,577 kJ/mol I5 = 14,125 kJ/mol Based on these relative values, which ionization energies correspond to removing core (nonvalence) electrons?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

You know the right answer?
An element has the following successive ionization energies: I1 = 578 kJ/mol I2 = 1817 kJ/mol I3 = 2...
Questions



Advanced Placement (AP), 07.12.2020 20:40

Biology, 07.12.2020 20:40

Biology, 07.12.2020 20:40

Mathematics, 07.12.2020 20:40





English, 07.12.2020 20:40

Physics, 07.12.2020 20:40


Mathematics, 07.12.2020 20:40

Mathematics, 07.12.2020 20:40

Biology, 07.12.2020 20:40




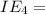 11,577 kJ/mol
11,577 kJ/mol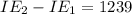 kJ/mol
kJ/mol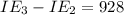 kJ/mol
kJ/mol kJ/mol
kJ/mol indicates the removal of core electron.
indicates the removal of core electron.

