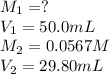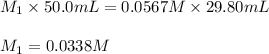Chemistry, 25.03.2020 00:28 itzdulceee
Acetic acid (CH3CO2H) is the principal component in the vinegar. What is the molarity of an acetic acid solution if a titration of 50.00 mL of the acetic acid solution requires 29.80 mL of 0.0567 M NaOH to reach the equivalence point?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Acetic acid (CH3CO2H) is the principal component in the vinegar. What is the molarity of an acetic a...
Questions

Advanced Placement (AP), 30.01.2021 06:00

Mathematics, 30.01.2021 06:00

Physics, 30.01.2021 06:00



SAT, 30.01.2021 06:00

Mathematics, 30.01.2021 06:00

Mathematics, 30.01.2021 06:00

English, 30.01.2021 06:00

Advanced Placement (AP), 30.01.2021 06:00

Mathematics, 30.01.2021 06:00

Mathematics, 30.01.2021 06:00

Mathematics, 30.01.2021 06:00


Mathematics, 30.01.2021 06:00

Biology, 30.01.2021 06:00




Mathematics, 30.01.2021 06:00

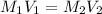
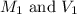 are the molarity and volume of acetic acid.
are the molarity and volume of acetic acid.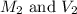 are the molarity and volume of NaOH.
are the molarity and volume of NaOH.