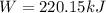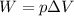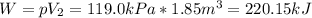Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
A gas‑forming reaction produces 1.85 m 3 1.85 m3 of gas against a constant pressure of 119.0 kPa. 11...
Questions




Mathematics, 21.07.2019 14:00

Computers and Technology, 21.07.2019 14:00




Chemistry, 21.07.2019 14:00


Computers and Technology, 21.07.2019 14:00


History, 21.07.2019 14:00

Computers and Technology, 21.07.2019 14:00


Chemistry, 21.07.2019 14:00

World Languages, 21.07.2019 14:00

Health, 21.07.2019 14:00

Chemistry, 21.07.2019 14:00