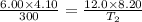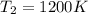Chemistry, 24.03.2020 23:00 charlesiarenee0
One mole of oxygen gas is at a pressure of 6.00 atm and a temperature of 27.0°C. (a) If the gas is heated at constant volume until the pressure triples, what is the fi nal temperature? (b) If the gas is heated so that both the pressure and volume are doubled, what is the fi nal temperature?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1


Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
You know the right answer?
One mole of oxygen gas is at a pressure of 6.00 atm and a temperature of 27.0°C. (a) If the gas is h...
Questions

Chemistry, 20.04.2021 18:50



French, 20.04.2021 18:50


Mathematics, 20.04.2021 18:50


History, 20.04.2021 18:50


Social Studies, 20.04.2021 18:50

Mathematics, 20.04.2021 18:50

Mathematics, 20.04.2021 18:50


Mathematics, 20.04.2021 18:50


History, 20.04.2021 18:50

Social Studies, 20.04.2021 18:50


Health, 20.04.2021 18:50

Mathematics, 20.04.2021 18:50

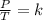
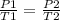
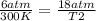
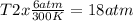
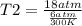
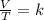
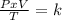
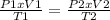
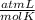 × 300 K
× 300 K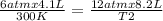
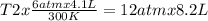
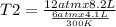
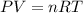
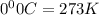
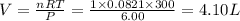
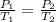
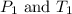 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.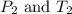 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.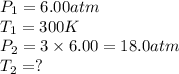
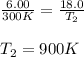
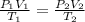
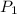 = initial pressure of gas = 6.00 atm
= initial pressure of gas = 6.00 atm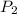 = final pressure of gas =
= final pressure of gas = 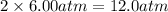
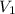 = initial volume of gas = 4.10 L
= initial volume of gas = 4.10 L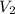 = final volume of gas =
= final volume of gas = 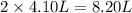
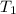 = initial temperature of gas =
= initial temperature of gas = 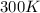
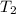 = final temperature of gas =?
= final temperature of gas =?