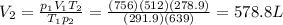Chemistry, 24.03.2020 22:15 namirah0303
A helium-filled weather balloon has a volume of 512 L at 18.9°C and 756 mmHg. It is released and rises to an altitude of 2.14 km, where the pressure is 639 mmHg and the temperature is 5.9°C.
The volume of the balloon at this altitude is
L.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
A helium-filled weather balloon has a volume of 512 L at 18.9°C and 756 mmHg. It is released and ris...
Questions


History, 18.10.2020 23:01




Mathematics, 18.10.2020 23:01


Computers and Technology, 18.10.2020 23:01


English, 18.10.2020 23:01

Mathematics, 18.10.2020 23:01

Mathematics, 18.10.2020 23:01

Mathematics, 18.10.2020 23:01

English, 18.10.2020 23:01




Social Studies, 18.10.2020 23:01

Biology, 18.10.2020 23:01

Advanced Placement (AP), 18.10.2020 23:01

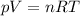
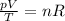

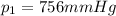 is the initial pressure of the gas
is the initial pressure of the gas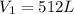 is the initial volume
is the initial volume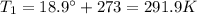 is the initial temperature
is the initial temperature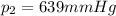 is the final pressure
is the final pressure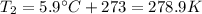 is the final temperature
is the final temperature