
You're provided with a bottle labled [CoCl2.6H2O] = 0.054 M in 4.60 M HCl. You heat a small volume of the solution in a hot water bath to 50 ∘C. If you determine that [CoCl42-] = 0.034 M at 50 ∘C at equilibrium, what must be the equilibrium concentration of [Cl-] at 50 ∘C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
You're provided with a bottle labled [CoCl2.6H2O] = 0.054 M in 4.60 M HCl. You heat a small volume o...
Questions


English, 19.04.2021 18:50

History, 19.04.2021 18:50




Biology, 19.04.2021 18:50


Mathematics, 19.04.2021 18:50

Mathematics, 19.04.2021 18:50


Physics, 19.04.2021 18:50



History, 19.04.2021 18:50

Mathematics, 19.04.2021 18:50

Mathematics, 19.04.2021 18:50



Mathematics, 19.04.2021 18:50

![[CoCl_2.6H_2O]](/tpl/images/0561/6622/227f6.png) = 0.054 M
= 0.054 M![[CoCl_4]^{2-}](/tpl/images/0561/6622/84955.png) = 0.034 M
= 0.034 M![[CoCl_2.6H_2O]+2HCl\rightarrow H_2[CoCl_4]+6H_2O](/tpl/images/0561/6622/defc4.png)
![[CoCl_2.6H_2O]+2Cl^-\rightarrow [CoCl_4]^{2-}+6H_2O](/tpl/images/0561/6622/500e9.png)
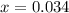
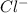 = (4.60 - 2x) = [4.60 - 2(0.034)] = 4.532 M
= (4.60 - 2x) = [4.60 - 2(0.034)] = 4.532 M

