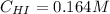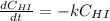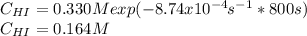Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys this rate law.
Rate= 8.74 x 10^-4 s^1
Suppose a vessel contains HI at a concentration of 0.330M. Calculate the concentration of HI in the vessel 800 seconds later. You may assume no other reaction is important. Round your answer to significant digit

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

You know the right answer?
Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys t...
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys t...
Questions



Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00



Biology, 19.02.2021 01:00



Biology, 19.02.2021 01:00

English, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

English, 19.02.2021 01:00

English, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00



English, 19.02.2021 01:00