

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3
You know the right answer?
The rate constant for the decomposition reaction of H2O2 is 3.66 × 10−3 s−1 at a particular temperat...
Questions






Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20


History, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

English, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20


Social Studies, 18.03.2021 03:20

Biology, 18.03.2021 03:20

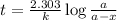

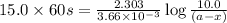
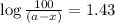
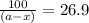
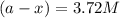
 in a solution after 15.0 minutes have passed is 3.72 M
in a solution after 15.0 minutes have passed is 3.72 M

