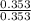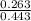A buffer solution is made that is 0.353 M in CH3COOH and 0.353 M in CH3COO- . (1) If Ka for CH3COOH is 1.80×10-5 , what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.090 mol HI is added to 1.00 L of the buffer solution. Use H3O+ instead of H+ .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
A buffer solution is made that is 0.353 M in CH3COOH and 0.353 M in CH3COO- . (1) If Ka for CH3COOH...
Questions



Chemistry, 29.11.2021 23:10

Computers and Technology, 29.11.2021 23:10

Physics, 29.11.2021 23:10







English, 29.11.2021 23:10

Social Studies, 29.11.2021 23:10


Mathematics, 29.11.2021 23:10



Mathematics, 29.11.2021 23:10

Computers and Technology, 29.11.2021 23:10

![\frac{[Salt]}{[Acid]}](/tpl/images/0561/5667/6743a.png)