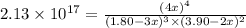Chemistry, 24.03.2020 21:33 BatmanVS1944
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, Kc. 3 A ( g ) + 2 B ( g ) − ⇀ ↽ − 4 C ( g ) K c = 2.13 × 10 17 If, at this temperature, 1.80 mol of A and 3.90 mol of B are placed in a 1.00 L container, what are the concentrations of A, B, and C at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3


Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium consta...
Questions

History, 03.02.2022 02:00












Arts, 03.02.2022 02:00

Mathematics, 03.02.2022 02:00


Biology, 03.02.2022 02:10

English, 03.02.2022 02:10

Business, 03.02.2022 02:10

Mathematics, 03.02.2022 02:10

![[A]=\frac{1.80 mol}{1.00 L}=1.80 M](/tpl/images/0561/5634/418e6.png)
![[B]=\frac{3.90 mol}{1.00 L}=3.90 M](/tpl/images/0561/5634/3de81.png)
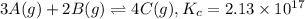
![K_c=\frac{[C]^4}{[A]^3[B]^2}](/tpl/images/0561/5634/6b95d.png)