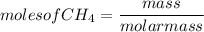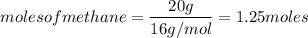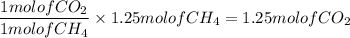Given the following reaction:
CH4 +202
CO2 + 2H2O
How many moles of CO2, will be prod...

Chemistry, 24.03.2020 21:11 cyaransteenberg
Given the following reaction:
CH4 +202
CO2 + 2H2O
How many moles of CO2, will be produced from 20.0 g of CH4, assuming O2 is available in excess?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1


Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
Questions


Biology, 16.07.2020 16:01

Mathematics, 16.07.2020 16:01


Mathematics, 16.07.2020 16:01

Mathematics, 16.07.2020 16:01


Health, 16.07.2020 16:01



Mathematics, 16.07.2020 16:01



Mathematics, 16.07.2020 16:01

Law, 16.07.2020 16:01


Mathematics, 16.07.2020 16:01