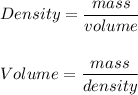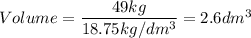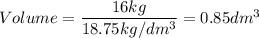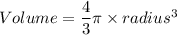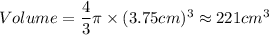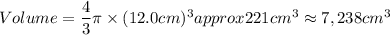Chemistry, 24.03.2020 19:58 AgarioEdit
A particular uranium alloy has a density of 18.75 g/cm3. Please answer the following questions below, providing the explanation to your answers. a. What volume is occupied by a critical mass of 49 kg of this alloy? b. The critical mass can be decreased to 16 kg if the alloy is surrounded by a layer of natural uranium (which acts as a neutron reflector). What is the volume of such smaller mass? Compare your answers to the approximate volumes of a baseball, a volleyball, and a basketball.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
A particular uranium alloy has a density of 18.75 g/cm3. Please answer the following questions below...
Questions


Mathematics, 31.07.2019 16:00


Mathematics, 31.07.2019 16:00






Biology, 31.07.2019 16:00




English, 31.07.2019 16:00




Biology, 31.07.2019 16:00