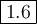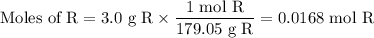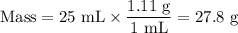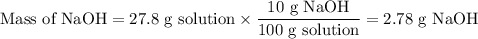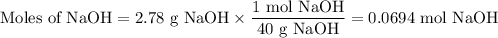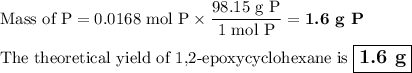Chemistry, 24.03.2020 20:15 soccerhannah290
The theoretical yield of 1,2-epoxycyclohexane is grams, when starting with 3.0 grams of trans-2-bromocyclohexanol. (Enter the number using 3 significant figures, i. e. 1.22) Given: 3.0 g of trans-2-bromocyclohexanol FW: 179.05 25 mL of 10% NaOH FW: 40 and density: 1.11 g/mL 1,2-epoxycyclohexane FW: 98.15

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
The theoretical yield of 1,2-epoxycyclohexane is grams, when starting with 3.0 grams of trans-2-bro...
Questions


Mathematics, 16.10.2020 14:01





Social Studies, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01

Social Studies, 16.10.2020 14:01


Computers and Technology, 16.10.2020 14:01

Biology, 16.10.2020 14:01






Mathematics, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01