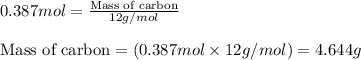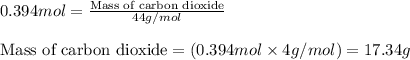For the following reaction, 9.37 grams of carbon (graphite) are allowed to react with 12.6 grams of oxygen gas . carbon (graphite)(s) + oxygen(g) carbon dioxide(g) What is the maximum mass of carbon dioxide that can be formed? grams What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete? grams Submit AnswerRetry Entire Group

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
For the following reaction, 9.37 grams of carbon (graphite) are allowed to react with 12.6 grams of...
Questions



Social Studies, 31.08.2019 13:00

Biology, 31.08.2019 13:00



Arts, 31.08.2019 13:00

Mathematics, 31.08.2019 13:00

Biology, 31.08.2019 13:00

Physics, 31.08.2019 13:00



Mathematics, 31.08.2019 13:00

History, 31.08.2019 13:00

Business, 31.08.2019 13:00


Health, 31.08.2019 13:00


Mathematics, 31.08.2019 13:00

Advanced Placement (AP), 31.08.2019 13:00

 and the mass of excess reagent (carbon) remained is 4.644 grams
and the mass of excess reagent (carbon) remained is 4.644 grams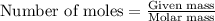 .....(1)
.....(1)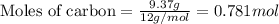
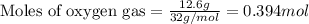
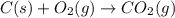
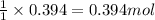 of carbon metal.
of carbon metal.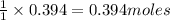 of carbon dioxide
of carbon dioxide