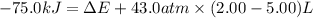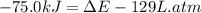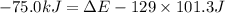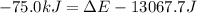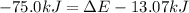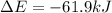Chemistry, 24.03.2020 19:30 leopolddc4006
Before the reaction, the volume of the gaseous mixture was 5.00 LL. After the reaction, the volume was 2.00 LL. Calculate the value of the total energy change, ΔEΔEDelta E, in kilojoules. Express your answer with the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Before the reaction, the volume of the gaseous mixture was 5.00 LL. After the reaction, the volume w...
Questions

Mathematics, 18.06.2020 01:57

Mathematics, 18.06.2020 01:57


Mathematics, 18.06.2020 01:57


Mathematics, 18.06.2020 01:57








Mathematics, 18.06.2020 01:57


Mathematics, 18.06.2020 01:57


Computers and Technology, 18.06.2020 01:57

Mathematics, 18.06.2020 01:57

Mathematics, 18.06.2020 01:57

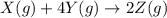 , ΔH=-75.0 kJ
, ΔH=-75.0 kJ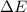 is, -61.9 kJ
is, -61.9 kJ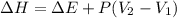
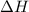 = change in enthalpy = -75.0 kJ
= change in enthalpy = -75.0 kJ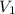 = initial volume of gas = 5.00 L
= initial volume of gas = 5.00 L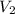 = final volume of gas = 2.00 L
= final volume of gas = 2.00 L