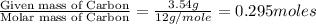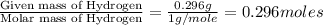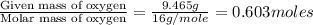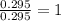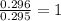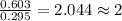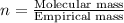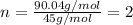Chemistry, 24.03.2020 19:38 cakeisalie6865
A 13.30 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis and 13.00 grams of CO2 and 2.662 grams of H2O are produced. In a separate experiment, the molar mass is found to be 90.04 g/mol. Determine the empirical formula and the molecular formula of the organic compound.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1
You know the right answer?
A 13.30 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis...
Questions

Mathematics, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00

English, 15.01.2021 14:00


History, 15.01.2021 14:00


History, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00

Chemistry, 15.01.2021 14:00

History, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00



Advanced Placement (AP), 15.01.2021 14:00



Computers and Technology, 15.01.2021 14:00

Biology, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00

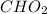 and
and 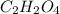
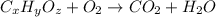
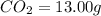
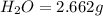
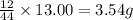 of carbon will be contained.
of carbon will be contained.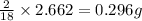 of hydrogen will be contained.
of hydrogen will be contained.