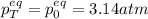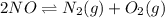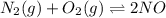Chemistry, 24.03.2020 19:22 brisamauro27
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the total pressure of the mixture at equilibrium is 3.14 atm . N 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO ( g ) K p = 0.101 at 2000 ° C In which direction does the reaction proceed after heating to 2000 °C? The reaction is at equilibrium. The reaction proceeds toward the reactants. The reaction proceeds toward the products.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the...
Questions



Chemistry, 31.12.2020 14:00

Arts, 31.12.2020 14:00



Business, 31.12.2020 14:00



Computers and Technology, 31.12.2020 14:00

Mathematics, 31.12.2020 14:00

Chemistry, 31.12.2020 14:00

Social Studies, 31.12.2020 14:00


Mathematics, 31.12.2020 14:00





Mathematics, 31.12.2020 14:00