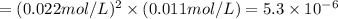Chemistry, 24.03.2020 19:20 ginachuquiano450
At a certain temperature (probably not 25 ºC), the solubility of silver sulfate, Ag₂SO₄, is 0.011 mol/L. Calculate its solubility product constant for this temperature. SIG. FIG. (required because number is small) Solubility product constants are very temperature sensitive. They are generally reported at 25 ºC. Not necessarily using this temperature allows me some flexibility.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
At a certain temperature (probably not 25 ºC), the solubility of silver sulfate, Ag₂SO₄, is 0.011 mo...
Questions


English, 25.07.2019 09:20



History, 25.07.2019 09:20

Spanish, 25.07.2019 09:20

Mathematics, 25.07.2019 09:20

Computers and Technology, 25.07.2019 09:20

Chemistry, 25.07.2019 09:20



Geography, 25.07.2019 09:20



Mathematics, 25.07.2019 09:20



Mathematics, 25.07.2019 09:20

Biology, 25.07.2019 09:20

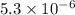 its solubility product constant for this temperature.
its solubility product constant for this temperature. 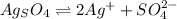
![[Ag_2SO_4]=0.011 mol/L](/tpl/images/0561/1517/1abc0.png)
![[Ag^+]=2\times [Ag_2SO_4]=2\times 0.011 mol/L = 0.022 mol/L](/tpl/images/0561/1517/4e43a.png)
![[SO_4^{2-}]=1\times [Ag_2SO_4]=1\times 0.011 mol/L = 0.011 mol/L](/tpl/images/0561/1517/739e3.png)
![K_{sp}=[Ag^+]^2[SO_4^{2-}]](/tpl/images/0561/1517/a7e5d.png)