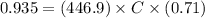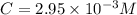Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1


Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Assume the molar absorptivity (ε) for CoCl42- at 700 nm is 446.9 M-1 cm-1. If d, the cell path lengt...
Questions

Mathematics, 11.01.2021 17:50

Mathematics, 11.01.2021 17:50


Mathematics, 11.01.2021 17:50


Mathematics, 11.01.2021 17:50

Mathematics, 11.01.2021 17:50

English, 11.01.2021 17:50

Mathematics, 11.01.2021 17:50

History, 11.01.2021 17:50

Geography, 11.01.2021 17:50


Biology, 11.01.2021 17:50

Mathematics, 11.01.2021 17:50




Health, 11.01.2021 17:50

Mathematics, 11.01.2021 17:50

Arts, 11.01.2021 17:50

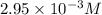

 = molar absorptivity coefficient =
= molar absorptivity coefficient =