
The reaction between carbon tetrachloride, CCl4, and water, H2O, to form carbon dioxide, CO2, and hydrogen chloride, HCl, has a ΔrG∘ value of −232 kJ mol−1, and so is thermodynamically favoured. But when you mix carbon tetrachloride with water, no change is observed. What is a possible explanation for this?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
The reaction between carbon tetrachloride, CCl4, and water, H2O, to form carbon dioxide, CO2, and hy...
Questions

English, 09.12.2020 20:50


Mathematics, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50




Mathematics, 09.12.2020 20:50




Mathematics, 09.12.2020 20:50


Chemistry, 09.12.2020 20:50

World Languages, 09.12.2020 20:50




Spanish, 09.12.2020 20:50

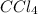 and
and 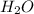 is thermodynamically favored due to negative enthalpy change but the high activation energy does not allow the reaction to take place by simple mixing.
is thermodynamically favored due to negative enthalpy change but the high activation energy does not allow the reaction to take place by simple mixing.

