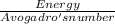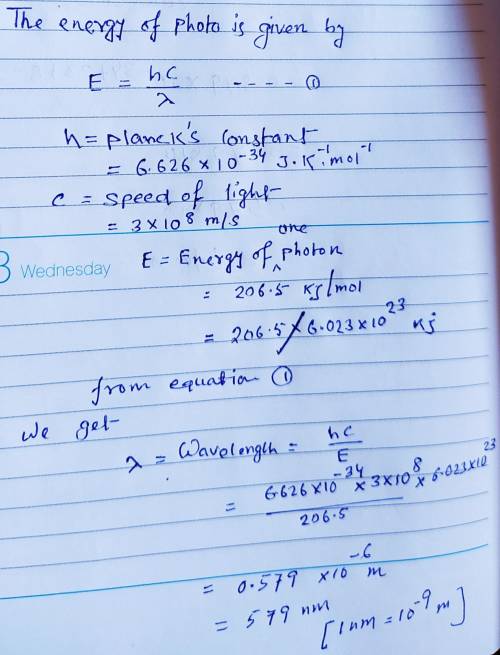Chemistry, 24.03.2020 17:54 orozcoalbeert
Cesium metal is frequently used in photoelectric cells because the amount of energy necessary to eject electrons from a cesium surface is relatively small-only 206.5 kJ/mol. Part A What wavelength of light (in nanometers) does this correspond to?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Cesium metal is frequently used in photoelectric cells because the amount of energy necessary to eje...
Questions

Mathematics, 23.07.2021 07:50

Mathematics, 23.07.2021 07:50

Mathematics, 23.07.2021 07:50

English, 23.07.2021 07:50


English, 23.07.2021 07:50

Mathematics, 23.07.2021 07:50

Mathematics, 23.07.2021 07:50

Health, 23.07.2021 07:50

Mathematics, 23.07.2021 07:50


Mathematics, 23.07.2021 07:50



English, 23.07.2021 07:50

Biology, 23.07.2021 07:50

History, 23.07.2021 07:50

History, 23.07.2021 07:50