
Chemistry, 24.03.2020 17:41 adriantheclashroyaln
A 32.5 g iron rod, initially at 22.4 ∘C, is submerged into an unknown mass of water at 63.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 59.7 ∘C. Part A What is the mass of the water? Express your answer to two significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
A 32.5 g iron rod, initially at 22.4 ∘C, is submerged into an unknown mass of water at 63.0 ∘C, in a...
Questions

Mathematics, 23.11.2021 03:50


Business, 23.11.2021 03:50


Mathematics, 23.11.2021 03:50


History, 23.11.2021 03:50

Mathematics, 23.11.2021 04:00





Mathematics, 23.11.2021 04:00

Mathematics, 23.11.2021 04:00


Mathematics, 23.11.2021 04:00


Mathematics, 23.11.2021 04:00

Mathematics, 23.11.2021 04:00

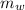 = 39.18 gm
= 39.18 gm = 32.5 gm
= 32.5 gm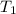 = 22.4°c = 295.4 K
= 22.4°c = 295.4 K = 0.448
= 0.448 

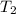 = 336 K
= 336 K  = 59.7°c = 332.7 K
= 59.7°c = 332.7 K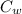 (
(

