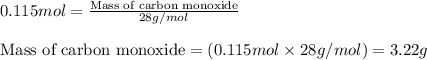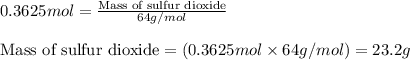Chemistry, 24.03.2020 16:34 micahatwood03
For the following reaction, 11.6 grams of sulfur are allowed to react with 23.5 grams of carbon monoxide . sulfur(s) + carbon monoxide(g) sulfur dioxide(g) + carbon(s) What is the maximum amount of sulfur dioxide that can be formed? grams What is the FORMULA for the limiting reagent? CO What amount of the excess reagent remains after the reaction is complete? grams

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
For the following reaction, 11.6 grams of sulfur are allowed to react with 23.5 grams of carbon mono...
Questions


Physics, 24.06.2019 07:50

English, 24.06.2019 07:50

Mathematics, 24.06.2019 07:50

Chemistry, 24.06.2019 07:50



Mathematics, 24.06.2019 07:50

Mathematics, 24.06.2019 07:50


Mathematics, 24.06.2019 07:50

Mathematics, 24.06.2019 07:50

Mathematics, 24.06.2019 07:50


Biology, 24.06.2019 07:50

Mathematics, 24.06.2019 07:50


History, 24.06.2019 07:50

Mathematics, 24.06.2019 07:50

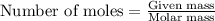 .....(1)
.....(1)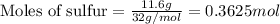
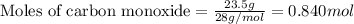
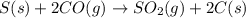
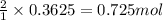 of carbon monoxide
of carbon monoxide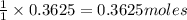 of sulfur dioxide
of sulfur dioxide