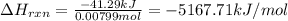Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8)(C10H8). When 1.025 gg of naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘C∘C to 32.33 ∘C Find ΔErxn for the combustion of naphthalene. The heat capacity of the calorimeter, determined in separate experiment, is 5.11kJ/∘C .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8)(C10H8). When 1.025 gg of nap...
Questions

History, 10.03.2020 00:14




Health, 10.03.2020 00:14




Computers and Technology, 10.03.2020 00:14





Mathematics, 10.03.2020 00:14


Mathematics, 10.03.2020 00:14




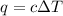
 = change in temperature =
= change in temperature = 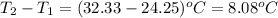
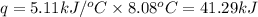
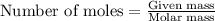
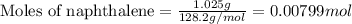
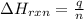
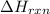 = enthalpy change of the reaction
= enthalpy change of the reaction