Chemistry, 24.03.2020 11:51 sydneyglover302
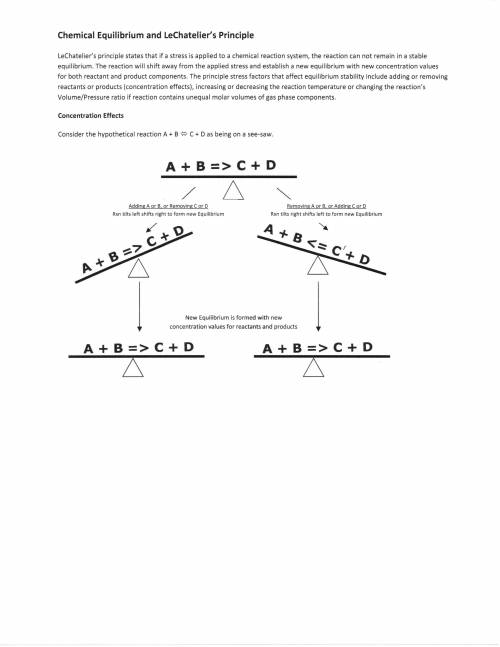
At equilibirium,1.0 liter vessel was found to contain 0.2moles,0.2moles B,0.4 moles C, and 0.4 moles D.İf 0.1 moles A and 0.1moles B added to system what will be the new equilibirium concentration of A?
A+B=C+D
reversable reaction

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
At equilibirium,1.0 liter vessel was found to contain 0.2moles,0.2moles B,0.4 moles C, and 0.4 moles...
Questions



Biology, 05.12.2021 21:30


Biology, 05.12.2021 21:30


Mathematics, 05.12.2021 21:30





Biology, 05.12.2021 21:30

English, 05.12.2021 21:30






Chemistry, 05.12.2021 21:30

Chemistry, 05.12.2021 21:30