
Chemistry, 24.03.2020 07:37 bridneyfondren
Calculate the molarity of a solution
made by 1,5kg of K. CO₂ in 850 mL
of water.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
Calculate the molarity of a solution
made by 1,5kg of K. CO₂ in 850 mL
of water....
made by 1,5kg of K. CO₂ in 850 mL
of water....
Questions


Health, 30.09.2019 22:00

English, 30.09.2019 22:00


Mathematics, 30.09.2019 22:00


Biology, 30.09.2019 22:00

Computers and Technology, 30.09.2019 22:00

Health, 30.09.2019 22:00


Mathematics, 30.09.2019 22:00

Health, 30.09.2019 22:00

Physics, 30.09.2019 22:00


Geography, 30.09.2019 22:00

Mathematics, 30.09.2019 22:00

Biology, 30.09.2019 22:00

Biology, 30.09.2019 22:00


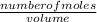
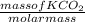
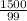 = 15.15moles
= 15.15moles 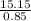 = 17.83M
= 17.83M

