
Chemistry, 24.03.2020 05:31 CurlyheadShay
Wrting an equilibrium constant for a reaction sequence Hydrogen is manufactured on an industrial scale by this sequence of reactions: CH,( H, O()Co()+3H2() co(g) +H2O(g) CO2(g) + H2(g) K, The net reaction is: CH4(g)+2H2O(g) CO2(g)+4H2(g) Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K and K If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

You know the right answer?
Wrting an equilibrium constant for a reaction sequence Hydrogen is manufactured on an industrial sca...
Questions


Mathematics, 06.06.2020 03:01

Mathematics, 06.06.2020 03:01

Chemistry, 06.06.2020 03:01

Mathematics, 06.06.2020 03:01

English, 06.06.2020 03:01


English, 06.06.2020 03:01

Mathematics, 06.06.2020 03:01



History, 06.06.2020 03:57


Mathematics, 06.06.2020 03:57


Mathematics, 06.06.2020 03:57


Mathematics, 06.06.2020 03:57

Mathematics, 06.06.2020 03:57

Social Studies, 06.06.2020 03:57

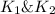 :
: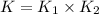
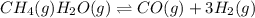
![K_1=\frac{[CO][H_2]^3}{[CH_4][H_2O]}](/tpl/images/0560/6092/ad23e.png)
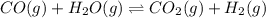
![K_2=\frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0560/6092/c2b62.png)
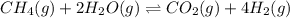
![K=\frac{[CO_2][H_2]^4}{[CH_4][H_2O]^2}](/tpl/images/0560/6092/3e51b.png)
![K=\frac{[CO_2][H_2]^4}{[CH_4][H_2O]^2}\times \frac{[CO]}{[CO]}](/tpl/images/0560/6092/b92b1.png)
![K=\frac{[CO][H_2]^3}{[CH_4][H_2O]}\times \frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0560/6092/2a778.png)


