
Chemistry, 24.03.2020 05:37 aROSSconpollo
A chemist designs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential (aq)(aq)(g)(l) (aq)(aq) Answer the following questions about this cell. a. Write a balanced equation for the half-reaction that happens at the cathode. b. Write a balanced equation for the half-reaction that happens at the anode. c. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. d. Do you have enough information to calculate the cell voltage under standard conditions

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
A chemist designs a galvanic cell that uses these two half-reactions: half-reaction standard reducti...
Questions

Mathematics, 07.04.2020 02:20


History, 07.04.2020 02:20



Mathematics, 07.04.2020 02:20

Physics, 07.04.2020 02:20


Biology, 07.04.2020 02:21




Mathematics, 07.04.2020 02:21







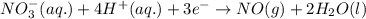 0.96 V
0.96 V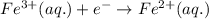 0.77 V
0.77 V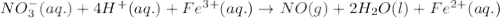
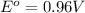
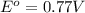
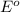 reduction potential will always get reduced and will undergo reduction reaction.
reduction potential will always get reduced and will undergo reduction reaction.![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[\text{Products}]}{[\text{Reactants}]}](/tpl/images/0560/6426/2f866.png)


