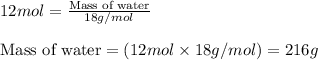Chemistry, 24.03.2020 02:44 musicaljay1276
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g) Which statement is correct about the complete combustion of 3.00 mole of propane, C3H8? \rm C_3H_8(g) + 5 O_2(g) --> 3 CO_2(g) + 4 H_2O(g)Which statement is correct about the complete combustion of 3.00 mole of propane, \rm C_3H_8?1. 12.00 mol H2O are produced.2. 3.00 g CO2 are produced.3. 3.00 mol CO2 are produced.4. 12.00 g H2O are produced

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

You know the right answer?
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g...
Questions

History, 31.01.2020 12:00

Mathematics, 31.01.2020 12:00

Health, 31.01.2020 12:00

Chemistry, 31.01.2020 12:00

Mathematics, 31.01.2020 12:00

History, 31.01.2020 12:00

Computers and Technology, 31.01.2020 12:00





Mathematics, 31.01.2020 12:00





Geography, 31.01.2020 12:00

History, 31.01.2020 12:00

Mathematics, 31.01.2020 12:00

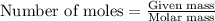 ......(1)
......(1)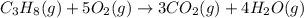
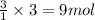 of carbon dioxide
of carbon dioxide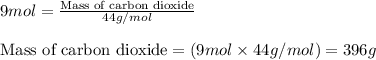
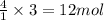 of water
of water