
Chemistry, 24.03.2020 02:26 johngayden46
Two stones resembling diamonds are suspected of being fakes. To determine if the stones might be real, the mass and volume of each are measured. Both stones have the same volume, 0.15 cm^3. However, stone A has a mass of 0.52 g, and stone B has a mass of 0.42 g.
A) If diamond has a density of 3.5 g/cm^3, could either of the stones be real diamonds? Explain.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1

You know the right answer?
Two stones resembling diamonds are suspected of being fakes. To determine if the stones might be rea...
Questions

Chemistry, 12.12.2020 16:20




Mathematics, 12.12.2020 16:20



Health, 12.12.2020 16:20


Chemistry, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

English, 12.12.2020 16:20

Arts, 12.12.2020 16:20

History, 12.12.2020 16:20

History, 12.12.2020 16:20



Mathematics, 12.12.2020 16:20


Chemistry, 12.12.2020 16:20

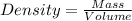
 .
. = 3.47
= 3.47  or after rounding off we get 3.5
or after rounding off we get 3.5  = 2.8
= 2.8 

