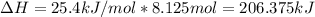Chemistry, 24.03.2020 02:26 ellaemtagedeane
Some instant cold packs contain ammonium nitrate and a separate pouch of water. When the pack is activated by squeezing to break the water pouch, the ammonium nitrate dissolves in water and the pack gets cold. The heat of solution for ammonium nitrate is 25.4 kJ/mol.
a) Is the dissolution of ammonium nitrate endothermic or exothermic?
b) A cold pack contains 135.0 g of water and 50.0 g of ammonium nitrate. What will be the final temperature of the activated cold pack, if the initial temperature is 25.0 degree C? (Assume that the specific heat of the solution is the same as that for water, 4.184 J/g degree C and no heat is lost).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
Some instant cold packs contain ammonium nitrate and a separate pouch of water. When the pack is act...
Questions

Mathematics, 03.02.2021 16:40

Mathematics, 03.02.2021 16:40

Social Studies, 03.02.2021 16:40

Mathematics, 03.02.2021 16:40


Mathematics, 03.02.2021 16:40

Mathematics, 03.02.2021 16:40






English, 03.02.2021 16:40

Mathematics, 03.02.2021 16:50


Mathematics, 03.02.2021 16:50



History, 03.02.2021 16:50