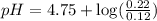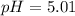Chemistry, 24.03.2020 02:29 doublejojo214
A solution is prepared by mixing 0.12 moles of acetic acid with 0.22 moles of sodium acetate in 1.00 liters of solution. What will be the pH of the solution once equilibrium is established?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
A solution is prepared by mixing 0.12 moles of acetic acid with 0.22 moles of sodium acetate in 1.00...
Questions

History, 18.03.2021 21:40





Computers and Technology, 18.03.2021 21:40


Mathematics, 18.03.2021 21:40

Business, 18.03.2021 21:40


History, 18.03.2021 21:40


Mathematics, 18.03.2021 21:40



History, 18.03.2021 21:40




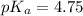
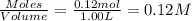
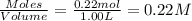
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0560/3046/e961a.png)