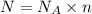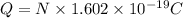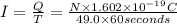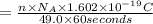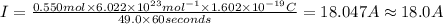Suppose 550.mmol of electrons must be transported from one side of an electrochemical cell to another in 49.0 minutes. Calculate the size of electric current that must flow. Be sure your answer has the correct unit symbol and round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
You know the right answer?
Suppose 550.mmol of electrons must be transported from one side of an electrochemical cell to anothe...
Questions

Biology, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30


Advanced Placement (AP), 03.12.2020 18:30

Mathematics, 03.12.2020 18:30

History, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30

Computers and Technology, 03.12.2020 18:30


Mathematics, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30


Mathematics, 03.12.2020 18:30



Mathematics, 03.12.2020 18:30