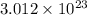Chemistry, 24.03.2020 01:27 juanesmania
When an ionic compound such as sodium chloride (NaCl) is placed in water the component atoms of the NaCl crystal dissociate into individual sodium ions (Na+) and chloride ions (Cl-). In contrast, the atoms of covalently bonded molecules (e. g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. Which of the following solutions would be expected to contain the greatest number of particles (molecules or ions)? A) 1 L of 0.5 M NaCl B) 1 L of 0.5 M glucose C) 1 L of 1.0 M NaCl D) 1 L of 1.0 M glucose E) C and D will contain equal numbers of particles.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1


Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
When an ionic compound such as sodium chloride (NaCl) is placed in water the component atoms of the...
Questions

Mathematics, 28.08.2020 17:01

Mathematics, 28.08.2020 17:01

Mathematics, 28.08.2020 17:01

Geography, 28.08.2020 17:01


Mathematics, 28.08.2020 17:01

Biology, 28.08.2020 17:01

Mathematics, 28.08.2020 17:01

Biology, 28.08.2020 17:01

Biology, 28.08.2020 17:01

Computers and Technology, 28.08.2020 17:01

Mathematics, 28.08.2020 17:01

History, 28.08.2020 17:01

Mathematics, 28.08.2020 17:01



Mathematics, 28.08.2020 17:01



English, 28.08.2020 17:01

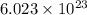 number of molecules/ions
number of molecules/ions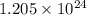 number of ions
number of ions volume) no. of particles
volume) no. of particles