
Chemistry, 24.03.2020 00:56 arielmajesty9107
The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(aq) + 5 I-(aq) + 6 H+(aq) --> 3 I2(aq) + 3 H2O(l) followed by reaction of the I2: I2(aq) + 2 S2O32- --> 2 I-(aq) + S4O62-(aq). What is the stoichiometric factor, that is the number of moles of Na2S2O3 reacting with one mole of KIO3?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(...
Questions





Arts, 16.04.2020 22:05


History, 16.04.2020 22:05




History, 16.04.2020 22:05




English, 16.04.2020 22:05

History, 16.04.2020 22:05




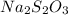 reacting with one mole of
reacting with one mole of 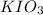 .
.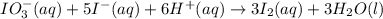 ..[1]
..[1]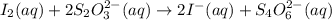 ..[2]
..[2]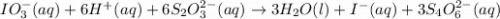
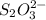 reacts with 1 mole of
reacts with 1 mole of 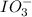 .
.

