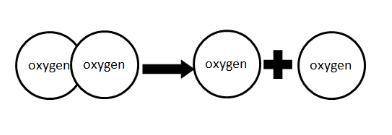
As we move from the left side of the arrow to the right, the two oxygen atoms exhibit
A) more...

Chemistry, 24.03.2020 00:31 lewisf2578
As we move from the left side of the arrow to the right, the two oxygen atoms exhibit
A) more stability.
B) higher entropy.
C) less potential energy.
D) a decrease in temperature.
IMAGE SHOWN BELOW.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1

You know the right answer?
Questions

Health, 24.01.2022 01:00



Chemistry, 24.01.2022 01:00


Mathematics, 24.01.2022 01:00

Mathematics, 24.01.2022 01:00


Mathematics, 24.01.2022 01:00


Mathematics, 24.01.2022 01:00


Mathematics, 24.01.2022 01:00

Mathematics, 24.01.2022 01:00





Mathematics, 24.01.2022 01:00

Mathematics, 24.01.2022 01:00



