
Chemistry, 24.03.2020 00:00 paolaviviana
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 10-8 at 700°C. 2 H2S(g) 2 H2(g) + S2(g) If 0.31 mol H2S is placed in a 4.1 L container, what is the equilibrium concentration of H2(g) at 700°C?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

You know the right answer?
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 10-8 at 700°C....
Questions




English, 03.09.2020 22:01

History, 03.09.2020 22:01


Mathematics, 03.09.2020 22:01


English, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

History, 03.09.2020 22:01


Mathematics, 03.09.2020 22:01



Mathematics, 03.09.2020 22:01


Mathematics, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

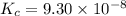 }
}![[concentration]=\frac{moles}{volume (L)}](/tpl/images/0559/9416/7e1bc.png)
![[H_2S]=\frac{0.31 mol}{4.1 L}=0.076 M](/tpl/images/0559/9416/b2f14.png)
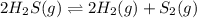
![K_c=\frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/0559/9416/3ac5e.png)
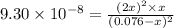
![[H_2]=2x=2\times 0.00051 M=0.0010 M](/tpl/images/0559/9416/46b67.png)


