
A 18.08-g sample of the ionic compound , where is the anion of a weak acid, was dissolved in enough water to make 116.0 mL of solution and was then titrated with 0.140 M . After 500.0 mL was added, the pH was 4.63. The experimenter found that 1.00 L of 0.140 M was required to reach the stoichiometric point of the titration. a What is the molar mass of ? Molar mass = 129.14 g/mol b Calculate the pH of the solution at the stoichiometric point of the titration.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3


Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2
You know the right answer?
A 18.08-g sample of the ionic compound , where is the anion of a weak acid, was dissolved in enough...
Questions



Mathematics, 12.10.2020 01:01



Computers and Technology, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01




Physics, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01

Spanish, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01

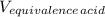 = 1.00 L
= 1.00 L
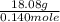
![10^{-4.63]](/tpl/images/0559/8018/9ece0.png)

![[A^-]equ = \frac{0.140M*1.00L}{1.00L+0.116L}](/tpl/images/0559/8018/a9cf4.png)
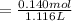
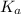 of HA =
of HA = 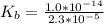

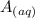 +
+ 
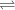
 +
+ 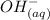
![K_b = \frac{[HA][OH^-]}{[A^-]}](/tpl/images/0559/8018/12da8.png)
![4.35*10^{-10} = \frac{[x][x]}{[0.1255-x]}](/tpl/images/0559/8018/ab7b7.png)
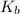 is very small, (o.1255 - x) = 0.1255
is very small, (o.1255 - x) = 0.1255

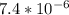 ]
]


