Chemistry, 23.03.2020 22:56 j1theking18
In a process for producing acetic acid, oxygen gas is bubbled into acetaldehyde, CH3CHO, containing manganese(II) acetate (catalyst) under pressure at 60°C. 2CH3CHO(l) + O2(g) → 2HC2H3O2(l) In a laboratory test of this reaction, 22.2 g CH3CHO and 12.6 g O2 were put into a reaction vessel. We wish to predict the following: a) How many grams of acetic acid can be produced by this reaction from these amounts of reactants?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
In a process for producing acetic acid, oxygen gas is bubbled into acetaldehyde, CH3CHO, containing...
Questions

English, 21.10.2020 05:01

Mathematics, 21.10.2020 05:01

History, 21.10.2020 05:01




Physics, 21.10.2020 05:01


Mathematics, 21.10.2020 05:01






English, 21.10.2020 05:01

Mathematics, 21.10.2020 05:01

English, 21.10.2020 05:01



English, 21.10.2020 05:01

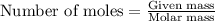 .....(1)
.....(1)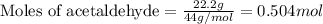
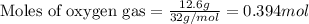
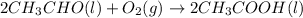
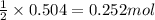 of oxygen gas
of oxygen gas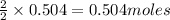 of acetic acid
of acetic acid