Chemistry, 23.03.2020 22:53 anthonybowie99
A calorimeter that measures an exothermic heat of reaction by the quantity of ice that can be melted is called an ice calorimeter. Consider a reaction in which 0.00400 mol of methane gas, CH4 (g), is burned completely at constant pressure in the presence of excess air. The heat liberated from the reaction melted 10.7 g of ice at 0 degrees celcius (the heat required to melt the ice (heat of fusion) is 333.5 J/g). What is the change in enthalpy for this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
A calorimeter that measures an exothermic heat of reaction by the quantity of ice that can be melted...
Questions

English, 28.01.2021 09:30


English, 28.01.2021 09:30


History, 28.01.2021 09:30




Social Studies, 28.01.2021 09:30





Mathematics, 28.01.2021 09:30

Mathematics, 28.01.2021 09:30

Mathematics, 28.01.2021 09:30

Mathematics, 28.01.2021 09:30



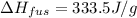
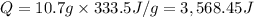
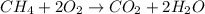 , ΔH = ?
, ΔH = ?