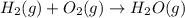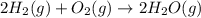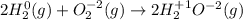Chemistry, 23.03.2020 22:28 Animallover100
In a hydrogen fuel cell, hydrogen gas and oxygen gas are combined to form water. Write the balanced chemical equation describing this reaction using the lowest whole‑number coefficients. classify each reaction as a redox reaction or a non‐redox reaction. redox non‐redox

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
In a hydrogen fuel cell, hydrogen gas and oxygen gas are combined to form water. Write the balanced...
Questions


Physics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

History, 15.12.2020 01:00


SAT, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00




Engineering, 15.12.2020 01:00

Physics, 15.12.2020 01:00


Arts, 15.12.2020 01:00



Mathematics, 15.12.2020 01:00


Arts, 15.12.2020 01:00