
A 100mL reaction vessel initially contains 2.60x10^-2 moles of NO and 1.30x10^-2 moles of H2. At equilibrium the concentration of NO in the vessel is 0.161M. At equilibrium the vessel contains N2, H2O and H2. What is the value of the equilibrium constant Kc for the following reactions?2H2(g) + 2NO(g) <---> 2 H2O(g) + N2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
You know the right answer?
A 100mL reaction vessel initially contains 2.60x10^-2 moles of NO and 1.30x10^-2 moles of H2. At equ...
Questions

Mathematics, 24.03.2021 02:20







Mathematics, 24.03.2021 02:20

Mathematics, 24.03.2021 02:20



Mathematics, 24.03.2021 02:20


Mathematics, 24.03.2021 02:20


Social Studies, 24.03.2021 02:20

Mathematics, 24.03.2021 02:20

Mathematics, 24.03.2021 02:20

Biology, 24.03.2021 02:30

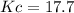
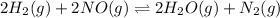
![[H_2]_0=\frac{1.30x10^{-2}mol}{0.1L}=0.130M](/tpl/images/0559/4541/78a1b.png)
![[NO]_0=\frac{2.60x10^{-2}mol}{0.1L}=0.260M](/tpl/images/0559/4541/9b7c0.png)
 , due to the chemical reaction extent, turns out:
, due to the chemical reaction extent, turns out:![x=\frac{[NO]_{0}-[NO]_{eq}}{2} =\frac{0.260M-0.161M}{2}=0.049M](/tpl/images/0559/4541/4c52a.png)
![[H_2]_{eq}=0.130M-2(0.049M)=0.032M](/tpl/images/0559/4541/cb7ca.png)
![[H_2O]_{eq}=2(0.049M)=0.098M](/tpl/images/0559/4541/63a65.png)
![[N_2]_{eq}=0.049M](/tpl/images/0559/4541/f2124.png)
![Kc=\frac{[H_2O]_{eq}^2[N_2]_{eq}}{[H_2]_{eq}^2[NO]_{eq}^2}=\frac{(0.098M)^2(0.049M)}{(0.032M)^2(0.161M)^2} \\\\Kc=17.7](/tpl/images/0559/4541/1d534.png)


