
Chemistry, 23.03.2020 21:11 copelandgarret9972
What concentration of Cl2O remains after a mixture that initially contains [H2O] = 1.00 M and [Cl2O] = 1.00 M comes to equilibrium at 25 °C ? Kc for the reaction is 0.0900.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
What concentration of Cl2O remains after a mixture that initially contains [H2O] = 1.00 M and [Cl2O]...
Questions

Mathematics, 02.12.2019 21:31

Biology, 02.12.2019 21:31







Mathematics, 02.12.2019 21:31

History, 02.12.2019 21:31


English, 02.12.2019 21:31



Mathematics, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31



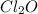 is 0.870 M.
is 0.870 M.![[H_2O]=1.00 M](/tpl/images/0559/4667/b0041.png)
![Cl_2O=[Cl_2O]=1.00 M](/tpl/images/0559/4667/4c14a.png)
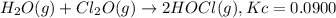
![K_c=\frac{[HOCl]^2}{[H_2O][Cl_2O]}](/tpl/images/0559/4667/da783.png)
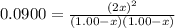
![[Cl_2O]=(1,00-x) M=1.00 M-0.130 M=0.870 M](/tpl/images/0559/4667/c9bd4.png)


