
Chemistry, 23.03.2020 20:21 ranaawilliamsoowl6dk
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemical equilibrium, which of the following explains what happens after the volume of the reaction mixture is increased (assume constant temperature)?
a. The amount of SO3(g) increases and the value for K increases.
b. The amount of SO3(g) decreases and the value for K increases.
c. The amount of SO3(g) stays the same and the value for K decreases.
d. The amount of SO3(g) decreases and the value for K stays the same.
e. The amount of SO3(g) increases and the value for K stays the same

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemica...
Questions

Physics, 10.10.2019 14:00

Mathematics, 10.10.2019 14:00

Mathematics, 10.10.2019 14:00

Mathematics, 10.10.2019 14:00


Mathematics, 10.10.2019 14:00


Social Studies, 10.10.2019 14:00


Mathematics, 10.10.2019 14:00

Mathematics, 10.10.2019 14:00

Mathematics, 10.10.2019 14:00

Mathematics, 10.10.2019 14:00







History, 10.10.2019 14:00

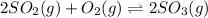
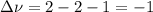
![K=\frac{[SO_3]_{eq}^2}{[SO_2]_{eq}^2[O_2]_{eq}}](/tpl/images/0559/3324/74387.png)


