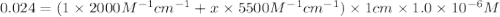The molar absorptivity of a tyrosine residue at 280 nm is 2000 M-1cm-1, while for tryptophan it is 5500 M-1cm-1. A protein has been isolated that is known to contain one tyrosine residue and an unknown number of tryptophans. A 1.0 micromolar solution of this protein is placed in a 1.0 cm cuvette and the absorbance at 280 nm is measured as 0.024. How many tryptophans are in the protein

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

You know the right answer?
The molar absorptivity of a tyrosine residue at 280 nm is 2000 M-1cm-1, while for tryptophan it is 5...
Questions

Arts, 24.01.2021 01:00

Mathematics, 24.01.2021 01:00

Mathematics, 24.01.2021 01:00




English, 24.01.2021 01:00

Mathematics, 24.01.2021 01:00

Mathematics, 24.01.2021 01:00

Advanced Placement (AP), 24.01.2021 01:00

English, 24.01.2021 01:00

Physics, 24.01.2021 01:00


History, 24.01.2021 01:00

History, 24.01.2021 01:00



Mathematics, 24.01.2021 01:00

Mathematics, 24.01.2021 01:00

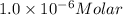


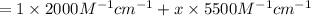
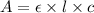 ( Beer-Lambert's law)
( Beer-Lambert's law)