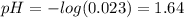Chemistry, 23.03.2020 19:27 nolangriffin
Arrange the following aqueous solutions, all at 25 ∘C, in order of decreasing acidity. 1 = most acidic; 4 = least acidic Rank from most acidic to most basic: 0.0018 M OH- 0.023 M H3O+ pH = 9.45 pOH = 5.52

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2


Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Arrange the following aqueous solutions, all at 25 ∘C, in order of decreasing acidity. 1 = most acid...
Questions

Advanced Placement (AP), 22.04.2020 05:29


Arts, 22.04.2020 05:29


History, 22.04.2020 05:29


Chemistry, 22.04.2020 05:30



Mathematics, 22.04.2020 05:30

Mathematics, 22.04.2020 05:30



Mathematics, 22.04.2020 05:30