
A reaction A ( aq ) + B ( aq ) − ⇀ ↽ − C ( aq ) has a standard free‑energy change of − 5.24 kJ / mol at 25 °C. What are the concentrations of A , B , and C at equilibrium if, at the beginning of the reaction, their concentrations are 0.30 M, 0.40 M, and 0 M, respectively?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
A reaction A ( aq ) + B ( aq ) − ⇀ ↽ − C ( aq ) has a standard free‑energy change of − 5.24 kJ / mol...
Questions

English, 13.04.2020 21:40



English, 13.04.2020 21:40


Mathematics, 13.04.2020 21:40



English, 13.04.2020 21:40



Mathematics, 13.04.2020 21:41

Mathematics, 13.04.2020 21:41

Mathematics, 13.04.2020 21:41




History, 13.04.2020 21:42

History, 13.04.2020 21:42

![[A]_{eq}=0.11M](/tpl/images/0559/0215/f777c.png)
![[B]_{eq}=0.21M](/tpl/images/0559/0215/4b396.png)
![[C]_{eq}=0.19M](/tpl/images/0559/0215/f0920.png)
![Kc=exp(-\frac{\Delta _RG }{RT} )=exp[-\frac{-5240J/mol }{(8.314J/mol*K)(298.15K)} ]=8.28](/tpl/images/0559/0215/70087.png)
 due to the chemical reaction, we obtain:
due to the chemical reaction, we obtain: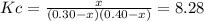
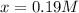
![[A]_{eq}=0.3M-0.19M=0.11M](/tpl/images/0559/0215/f33fb.png)
![[B]_{eq}=0.4M-0.19M=0.21M](/tpl/images/0559/0215/a81c9.png)


